There are two main types of tests: Molecular and Serological.
Molecular diagnostics describes testing to detect specific sequences in DNA or RNA and can be specific for the substance being tested. These are the first type of tests being used by the World Health Organization (WHO) and Center for Disease Control (CDC) to identify the virus that causes COVID-19 in respiratory specimens.
State and local public health departments have received these tests from CDC, whereas medical providers are getting tests developed by commercial manufacturers. They detect viral genetic material to show whether the coronavirus has infected that person. Until April 13, the only approved molecular test required a swab from deep in the nasal passages or the back of the throat. A negative result with the molecular test means that the virus that causes COVID-19 was not found in the sample (above the limit of detection), but it is still possible to have very low levels of the virus in the body. It does not indicate if the patient has developed any immunity or if they have recovered from the virus.
There is a massive focus by life science companies to continue to develop (molecular) diagnostic tests for the virus as well as for (serological) antibody tests to determine if people have been exposed and have immunity to the disease.
Nasal molecular diagnostic tests have unfavorable characteristics public and private companies are working hard to resolve:
- Process is uncomfortable requiring a deep swab into the nasal passage by inserting a 6-inch long swab into the back of the one nostril and rotating the swab several times for 15 seconds. The process is repeated in the other nostril and usually elicits a cough from the person being tested.
- Introduces exposure for the tester to the virus- requiring the tester to use critically needed protective wear.
- If the test is not completed by a trained medical person, there is high potential for being performed improperly and result in a false negative.
- Testing kits require materials that are short supply including swabs.
- The testing process and time to get results takes hours to days given the volume, resulting in missed work, or unnecessary isolation. (Lab Corp, the nation’s largest lab suggests on their website it takes 2-4 days after picking up a specimen to process).
The above stated are the reasons the CDC is only recommending testing symptomatic people. But there is confusion with media reports and advertisements which have led some to believe testing is available across the country for anyone who wants it. To further baffle the public, start-up companies have been marketing “at-home test kits” available for anyone to purchase, but the FDA states (March 20) these kits are “fraudulent” and are not proven to work.
Even Walgreen’s and C.V.S. advertise having testing available. However, reading the fine print, the advertisements on both websites state, “Because of limited supplies and in accordance with CDC guidelines, testing is limited to high-risk patients.” The sites further require the patient complete an approval process to set appointment.
Saliva Molecular Testing was approved on April 13, 2020 by the Food and Drug Administration. The FDA granted emergency use authorization (EUA) to Rutgers’ RUCDR Infinite Biologics for its test that uses saliva. This method could allow broader population screening for active coronavirus infections—however, the FDA’s green light covers the prescription-only test’s use at the university’s New Jersey lab for the time being. Developed in collaboration with Accurate Diagnostic Labs and utilizing Spectrum DNA’s spit collection and preservation kits, the researchers were able to show their saliva-based test performed just as well as the potentially painful swabs when searching for the specific RNA sequences linked to the novel coronavirus.
By using saliva, risk is reduced to spreading the virus to the health care professionals, precious personal protective equipment is preserved and the ability to increase the number of people tested is suddenly scaled. The Saliva Test science is based on a laboratory technique that makes millions of copies of the COVID-19 nucleic acid from a single sample and can potentially allow for processing of tens of thousands of samples a day. And yes, it will mean people will only need to spit in a tube. The Saliva Test is being deployed in New Jersey this week.
Serological Testing also known as antibody testing is the second form mentioned most in the news. Commercial serological tests (antibody tests) are being developed globally but their reliance for diagnosis is varying. The U.S. Food and Drug Administration (FDA) approved the first blood test for antibodies against COVID-19 on April 1, 2020 and the emergency approval will remain in effect for the duration of the pandemic. The company, Cellex, (Research Triangle Park, North Carolina) received authorization to begin using the blood test (Cellex qSARS-COV-2 IgG/IgM Rapid Test) in April.
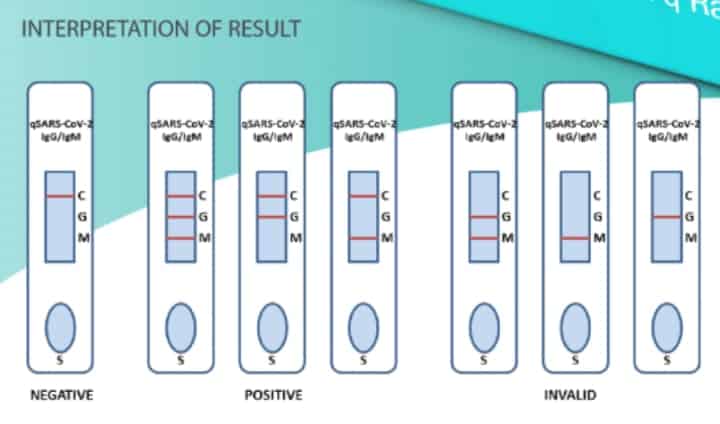
The variability in serological tests (antibody tests) is related to the days for an individual’s immune system to attack the virus and produce the antibodies. Some people have taken up to 11 days to develop antibodies and therefore these tests aren’t used to diagnose patients with recent (2-3 days) of symptoms of COVID-19. Serological testing gives a more comprehensive view of the person but does not detect viral genetic material to show whether the coronavirus has infected that person, rather it shows the antibodies in the blood to fight the infection. According to Cellex website, the takes around 15 to 20 minutes to get a result.
Cellix – Interpretation Guide for Antibody Test:
Inspired Living’s goal is to keep our residents safe during this pandemic. Given the current challenges with testing, the best way to do that is continuing to reinforce washing hands, maintain heightened cleaning and screening standards, practice social distancing and monitoring residents for symptoms.
For answers to visitation questions specific to your Inspired Living community, please contact the community’s executive director.